Abstract
Introduction: A change in TKIs is needed in 30-50% of CP-CML patients due to adverse events or suboptimal response/treatment failure. With 5 TKIs Currently available: imatinib (IM), dasatinib (DAS), nilotinib (NIL), bosutinib (BOS), and ponatinib (PON) we sought to evaluate outcomes of CP CML following sequential TKI treatment.
Method: Record of CP-CML referred to our center were reviewed. Patients who did not initiate TKI within 6 months after diagnosis were excluded.
Results: Between January 2005 and April 2016, 206 patients with CP CML were identified. Clinical characteristics are summarized in table 1. With a median follow-up of 48.4 (1.4-183.7) months 36.9% received 1 TKI, 35.9% 2 TKIs and 27% received > 3 TKIs. Eighteen patients (8.7%) reused same TKIs in different treatment lines. IM was the most frequently used in 1st line treatment (n=145, 70.4%), DAS in 2nd line treatment (n=68, 52.3%), NIL in 3rd line treatment (n=21, 32.8%), and PON in 4th and 5th line treatment (n=10, 34.5%, and n=4, 40%), respectively. Reason for switching TKIs included resistance (50%), intolerance (43.3%), and others (insurance, clinical trial, or reason are not available, 6.7%). ABL domain mutations were detected in 25 patients (12.1%), of which 9 were T315I.
Overall, 19 (9.2%) progressed to advanced phase (AP, n=6; myeloid BC (MBP), n=4; lymphoid BP (LBP), n=9). Eight patients underwent allogeneic HSCT due to TKI failure (n=2), presence of T315I mutation (n=1), irreversible IM-associated neutropenia (n=1), and progression to LBP (n=3) and AP (n=1). One underwent an autologous HSCT due to refractory leukemic meningitis. Ten patients expired because of complication of allogenic HSCT (n=3), refractory disease (n=6) and acute myocardial infarction (n=1).
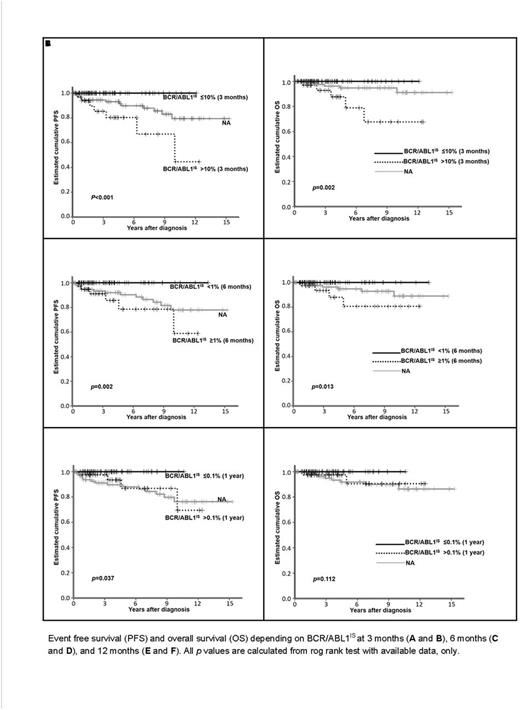
Estimated cumulative PFS at 5- and 10-year were 90% and 80%, and OS at 5- and 10-year were 94% and 90% respectively for the entire group. Curves depicting PFS and OS according to number of TKI exposure, are represented in the figure. OS at 10 years for patients exposed to 1 TKI was 100% (n=76), 2TKI, 95% (n=74), 3TKIs, 85% (n=33), 4 TKIs, 73% (n=16), and 5TKIs, 55% (n=7), respectively (p=0.001). Conclusion: CP CML tolerating and responding to first and second-line TKI had the best survival but survival of patients exposed to up to 5 TKIs was acceptable (10 years OS of 55%), suggesting that sequential use of TKI may be a reasonable approach in CP-CML.
Heffner: ADC Therapeutics: Research Funding. Arellano: Cephalon Oncology: Research Funding. Kota: Pfizer: Consultancy; Novartis: Consultancy; Xcenda: Consultancy; Incyte: Consultancy; Takeda Pharmaceuticals: Consultancy; Leukemia Lymphoma Society: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal